Learning how to calculate density is tricky, but we intend to make this much easier than you ever thought! Calculating density can be problematic depending on the state of matter involved. The measurement of certain physical properties related to density can be practiced differently depending on whether it is done for a solid, liquid, or gaseous aggregate state. This does not make it a problem to implement an effective methodology for calculating density regardless of the exact aggregate state in question, as long as we have their physical characteristics available.
Formula For Calculating Density
To calculate the density of a certain material, we must know the following characteristics:
- The mass of the material.
- The volume of the material.
Before presenting the formula for calculating density, let’s think a little about how the density of a certain substance is related to its mass and volume.
In the first situation, imagine two bodies that have the same volume but different mass. Logically, a body that has more mass must be denser, while a body that has less mass must have less density!
In the second situation, imagine two bodies that had the same mass but different volumes. Logically, a body that has a smaller volume will be denser, while a body that has a larger volume must have a lower density.
These two conclusions can help us create the density calculation formula. Density depends directly on the mass, while it depends inversely on the volume of the material whose density we want to know.
To calculate the density of a physical body or material, it is enough to divide its mass by its volume! The formula for calculating density looks like this:
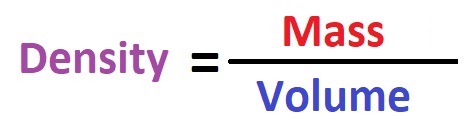
We can present the formula in a short form (with physical symbols) as follows:
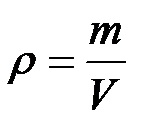
Any reader can easily figure out what the mass, density and volume symbols are!
Examples On How To Calculate Density
Finally, let’s see the density calculation formula in action and see how it applies to specific situations. We suggest you take a look at the solved examples below on this page!
Example 1: Determine the density of a body that has a mass of 540 kilograms, if its volume is 0.45 cubic meters!
In the formula, we substitute 540 kilograms in the place of mass, while we substitute 0.45 cubic meters in the place of volume. This substitution yields the following expression:
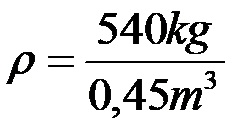
Finally, it is enough to calculate the quotient between the numbers 540 and 0.45 to determine the density of the body!
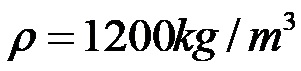
Finally, we find that the body has a density of 1200 kilograms per cubic meter.
Example 2: Calculate the density of water with a volume of 200 cubic centimeters, if its mass is 200 grams.
In the formula, we substitute 200 grams for mass, while we substitute 200 cubic centimeters for volume. This substitution yields the following expression:
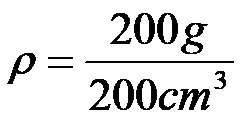
Finally, it is enough to calculate the quotient between the numbers 200 and 200 to determine the density of water!
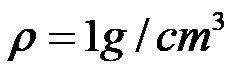
Finally, we state that water has a density of 1 gram per grain per cubic centimeter.
Density Measures
To calculate density you must use appropriate measures of mass and volume. Good combinations to calculate are:
- Kilogram for mass, cubic meter for volume.
- Gram for mass, cubic centimeter for volume.
If either of these two combinations is not observed then you can very easily get a measure of density that will not be understandable to people, such as kilogram per cubic hectometre. Therefore, if the initial given mass or volume measurements do not match the above combinations, it is you who must make the appropriate adjustments before starting to calculate density!
We hope you had fun learning how to calculate density on this page of ours. If this is the case, we recommend that you browse our entire website. Also feel free to send us any requests for new material you think we should cover in the future! Thank you!




